High-dose EPA for Long Covid & ME/CFS?
Could a prescription-strength formulation of the omega-3 fatty acid help long-haulers and those with ME/CFS?
Recently, I spoke with an MD who has treated approximately 40 Long COVID patients with a prescription strength omega-3 fatty acid similar to EPA known as icosapent ethyl (IPE)— with promising results. Even patients who had suffered from Long COVID for over two years reportedly experienced improvements in sense of taste & smell, fatigue, sleep, joint pain, brain fog, and hair/nail growth. You can read the case series here and also here. One of the case reports is quoted below. NOTE: Vascepa is a brand name of IPE, and neither I nor the authors of the cited case series have affiliation with the company that manufactures Vascepa.
This MD also treated about 140 acute COVID patients with Vascepa and reportedly none of them developed Long COVID. The MITIGATE trial is currently investigating the ability of Vascepa (IPE) to reduce morbidity and mortality in a cohort of adults with upper respiratory infections including SARS-CoV-2. The authors of this study state: “Preclinical data and clinical observations suggest that IPE may have pleiotropic effects including antiviral and anti-inflammatory properties that may prevent or reduce the downstream sequelae and cardiopulmonary consequences of viral URIs.”
One naturally wonders if IPE may also prevent or at least attenuate the development of Long COVID. When I reached out to the study authors to inquire if they are also looking into this, however, I received no response.
The case series motivated me to dig deeper into the role of essential fatty acids (EFA) in both ME/CFS and Long Covid. First, a brief review: Alpha-linolenic acid (ALA) is an essential omega-3 fatty acid, while linoleic acid (LA) is an essential omega-6 fatty acid. These EFAs must be converted to their active metabolites by an enzyme known as ∆-6-desaturase. For ALA, those metabolites include EPA and DHA; for LA, they include GLA and arachidonic acid. See below for more details (source for image here).
It turns out that research in this sphere goes back decades:
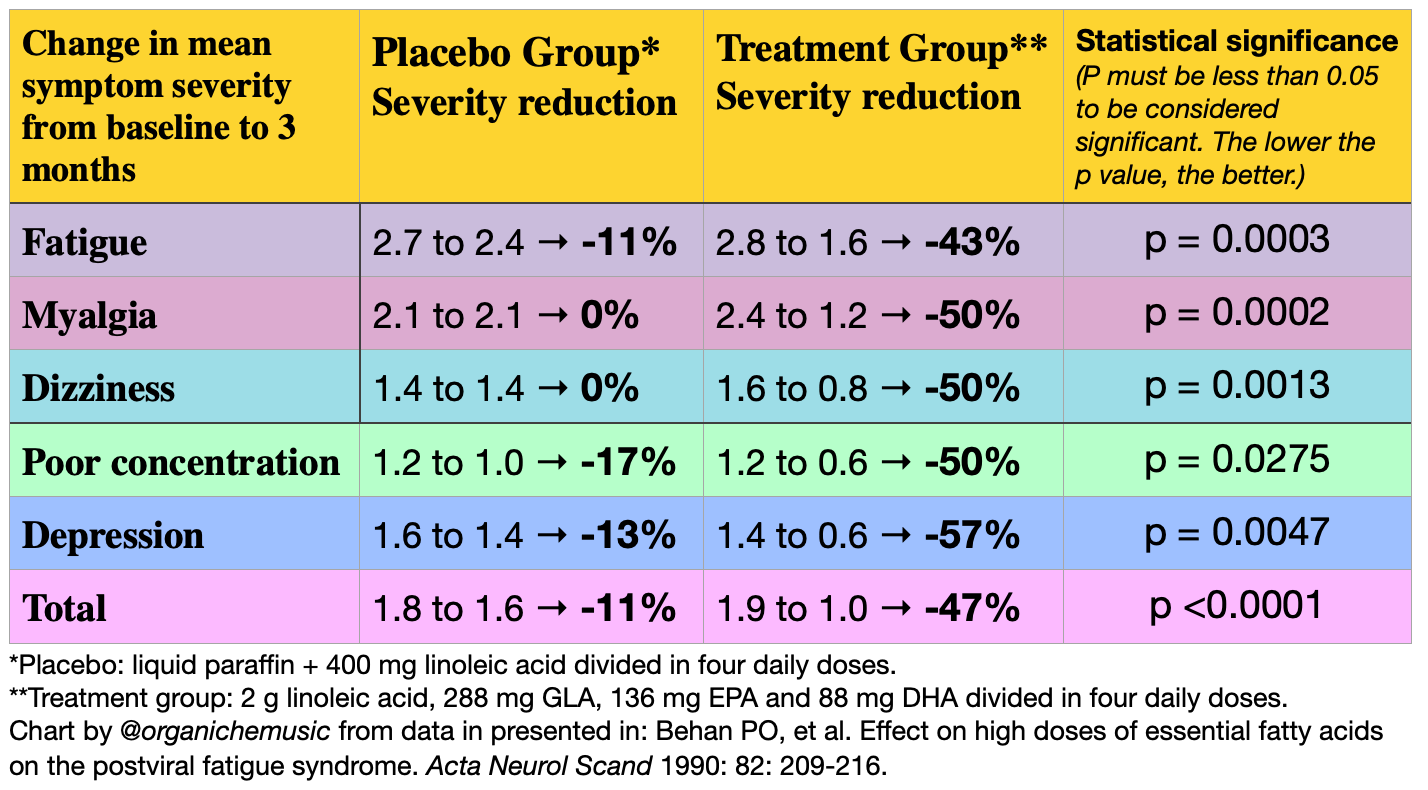
Study #1:
A 1990 double-blind, placebo-controlled trial treated 63 post-viral fatigue syndrome patients with high-dose EFA. The 27 men & 36 women were on average 40 years old and had suffered from post-viral fatigue syndrome for 1 to 3 years. Their symptoms were similar to those in Long Covid and ME/CFS: overwhelming fatigue exacerbated by exercise, myalgia, dizziness, palpitations, chest pain, brain fog, etc. Twenty-four patients were given placebo while thirty-nine were given a three-month trial of 2 g linoleic acid, 288 mg GLA, 136 mg EPA and 88 mg DHA divided into four daily doses. 85% vs 17% in the treatment & placebo groups reported benefit, respectively, while 0% vs 9% reported feeling worse. Symptoms were assessed at baseline, 1 month, and 3 months (1 = mild, 2 = moderate, 3 = severe). Myalgia improved the most quickly at 1 month, while all symptoms monitored improved significantly after 3 months compared to placebo:
Study #2:
In 1999, an attempt was made to replicate the above study, but this second iteration seemed inferior. The sample size was smaller (50 vs 63), and while the same active treatment and dosage were used, the authors made the strange choice to use a “placebo” that contained 2.7 grams of linoleic acid. No statistically significant differences were found between the treatment group and the “control” group. Frustratingly, I am only able to access the study abstract so cannot further assess.
Study #3
A small case series published in PLEFA in 2004 investigated the use of EPA in the treatment of four “chronic, intractable fatigue syndrome” patients. These patients took the 5 to 9 capsules twice daily of the supplement “eye q.” Each capsule contained 93 mg EPA, 29 mg DHA, & 10 mg GLA and the total daily dose of EPA ranged from almost 1 to 2 grams. The patients reported significant benefits, but disappointingly, no objective data was collected. Improvements reportedly began within 12 weeks of treatment.
The author of this study, Dr. Basant Puri, also published a book on treating ME/CFS with EPA. In his book he suggests that patients take over 2 grams of highly-purified EPA along with 800 mg of evening primrose oil both in two divided doses. He advises against DHA supplementation, claiming it is more prone to oxidation (rancidity, which I will discuss more below). Dr. Puri hypothesizes that low-grade viral persistence inhibits the enzyme ∆-6-desaturase which is critical for EFA conversion to active metabolites like EPA & DHA as mentioned above. Past in vitro studies found that viruses do indeed inhibit this enzyme. In fact, when viruses inhibit ∆-6-desaturase, it may cause a downstream effect that ultimately reduces the release of interferons—crucial signaling proteins that warn the body of unwanted viral presence and trigger an immune response.
This hypothesis seems plausible, but it doesn’t explain why patients in Study #1 improved as well as they reportedly did on linoleic acid. Recall that linoleic acid is an essential omega-6 fatty acid that requires ∆-6-desaturase to convert to active fatty acid metabolites, and patients took high doses of linoleic acid vs relatively low doses of the active metabolites EPA, GLA and DHA. If their bodies indeed could not convert linoleic acid to active metabolites due to ∆-6-desaturase inhibition, it is surprising that they benefitted a well as they reportedly did. Perhaps this is one reason why the replicated version of that study had more disappointing results.
MY THOUGHTS:
Considering the above trials, case series, and further readings, I suspect that high-dose EPA or its derivative IPE may be a key to improvement in some ME/CFS and Long COVID sufferers. Furthermore, I suspect that those who have been supplementing with OTC fish oils may have missed out on potential benefits due to the following:
poor supplement quality
suboptimal dosing, and/or
ineffective DHA/EPA ratios
SUPPLEMENT QUALITY
Earlier this year, Labdoor found that 10% of the fish oil supplements they tested were rancid, with almost 50% just barely under the recommended maximum limit. Alarmingly, a 2015 study of 32 fish oil supplements in New Zealand uncovered that 50% exceeded recommended total oxidation levels and only 8% met international recommendations. A North American analysis of 171 omega-3 polyunsaturated fatty acid (PUFA) supplements found that “50 % [of those successfully tested] exceeded the voluntary recommended levels for markers of oxidation.” Disturbingly, children’s omega-3 PUFA supplements revealed even higher oxidation levels, and the greater the rancidity, the more flavorings companies used to mask the taste. Look for brands that are transparent about their product source, total oxidation values, and heavy metal & PCB levels like Nordic Naturals.
UPDATE: I have learned that the Guardian article is misleading. It turns out that added flavoring/colors in fish oil result in false positives for anisidine levels, thus artificially increasing the TOTOX (total oxidation) values in some fish oil supplements. For example, the Guardian article cites Carlson cod liver oil with a hugely elevated TOTOX of 280. However, this product contained flavorings and thus the level is unreliable. Here is the certificate with TOTOX values for their EPA Elite Gem which does not contain flavorings and is quite low at 6.5— in the same range as Nordic Naturals typical TOTOX values and well under the recommended level of 26. This is good news, but it's still advisable to ask companies for TOTOX before deciding whether or not to purchase. If the company is evasive, that's a red flag.
SUBOPTIMAL DOSING
In the Long COVID case studies cited above, patients improved with Vascepa (IPE) mostly in the 3 to 4 grams daily dosage range. Dr Puri recommends over 2 grams daily: his patients typically take 8 capsules of VegEPA, a DHA-free supplement that offers 280 mg EPA per capsule. However, most over-the-counter (OTC) supplements contain around 200 to 400 mg of EPA per gelcap along with a similar dose of DHA — that’s only 5% to 20% of the recommended EPA or IPE dose! Here’s an example of a typical brand:
There are a few more expensive brands that include higher doses of EPA, but these formulations include some DHA. Unfortunately, addition of DHA may actually counteract or reduce benefits of EPA…
EPA/IPE MONOTHERAPY vs. EPA/DHA COMBO
Multiple trials have found significant cardiovascular benefits with EPA or IPE monotherapy but no significant benefits with EPA/DHA combo therapy. In 2018, the REDUCE-IT trial (n = 8,179 ) concluded that IPE 2 g twice daily significantly improved cardiovascular morbidity and mortality in the studied population compared to placebo (25% composite reduction). On the other hand, the STRENGTH trial (n=13,078) found *no benefit* in a similar cohort who took the same dose of a EPA/DHA combo. In fact, investigators stopped the trial early because it showed no promise. Thus while IPE 4 grams daily showed clear cardiovascular benefit, the same dose of EPA with DHA did not. This is consistent with prior trials and highlights the fact that DHA may be counteracting beneficial effects of EPA…
EPA vs DHA
Past studies have illustrated that EPA and DHA may compete against each other and exert different effects. One intriguing meta-analysis investigated the efficacy of DHA/EPA supplementation in treating depression. Authors found that improvement correlated with dose of EPA in excess of DHA: EPA/DHA combos with ≥60% EPA improved depression on average by over 55% while lower ratios had no effect or even worsened depression.
Some particularly pertinent benefits of omega-3 fatty acids in ME/CFS and Long COVID — half of which are unique to and/or superior with EPA— are listed below.
Nitric Oxide (NO) promotion: EPA promotes vascular regrowth in part due to induction of nitric oxide release (NO). This study found that “EPA significantly improved NO bioavailability in human endothelial cells compared to DHA and arachidonic acid (AA).” Furthermore, EPA reduced peroxynitrite, a reactive nitrogen species that can cause vascular dysfunction (see chart below).
Cell membrane stabilization: An X-ray diffraction study found that EPA and DHA exert different effects on the lipid bilayer of cell membranes. EPA readily incorporates into the cell membrane core and stabilizes it, whereas DHA does not:
Why does this matter? Cell membranes are essential for cellular function: not only do they provide structural support for cells, but they also facilitate cell-to-cell communication and nutrient/toxin transport. Different effects of EPA and DHA on membrane stability likely elicit different effects in cell signaling. A second study revealed that in addition to stabilizing cell membranes, EPA is also protective against harmful reactive oxygen species and lipid peroxidation.
Immunomodulatory benefits: Omega-3 fatty acids affect both innate and adaptive immune responses. Examples of innate immune cells include mast cells, neutrophils, NK cells, and macrophages, while adaptive immune cells include T and B lymphocytes. These cells release cytokines and chemokines to coordinate appropriate immune responses. A recent study discovered that the omega-3 index is significantly and inversely associated with the neutrophil-lymphocyte ratio, a biomarker for not only inflammation but also innate-adaptive immune system balance. However, authors of this study did not distinguish between EPA and DHA levels.
Anti-inflammation: In a systematic review of four RCTs with 274 COVID patients, omega-3 fatty acids significantly reduced C-reactive protein (CRP, an inflammation biomarker) compared to the control group which did not receive omega-3 supplementation. A meta-analysis found that both EPA and DHA similarly reduce CRP, IL-6, and TNF-α. However, another meta-analysis found that EPA seemed to reduce CRP to a greater extent than DHA.
WRAPPING UP: EPA in Long COVID and ME/CFS
1 to 2 grams twice daily of IPE or EPA for at least 12 weeks may be beneficial in some people with Long COVID or ME/CFS, improving symptoms such as poor sleep, brain fog, exercise intolerance, smell/taste, & hair/nails. Some info on brands to consider is shown in the chart below:
CAUTIONS:
Atrial fibrillation
In the REDUCE-IT trial, a significantly larger percentage of patients in the IPE group vs placebo group were hospitalized for atrial fibrillation or flutter (3.1% vs. 2.1%, P=0.004). Overall, 5.3% (IPE) vs 3.9% (placebo) experienced AFib or AFlutter. A meta-analysis of seven omega-3 fatty acid studies found that heightened risk of AFib is not limited to just IPE, but any omega-3 fatty acids. If you notice a worsening of heart palpitations, fatigue and/or shortness of breath, contact your doctor to be evaluated. Those with a history of AFib or atrial flutter should speak with their doctor before starting EPA ≥ 1 g daily.
Serious bleeding events occurred in 2.7% and 2.1% of the patients in the IPE and placebo, groups, respectively, which was not statistically significant (P=0.06).
If you have a fish allergy, please be advised the products listed above are all derived from fish.
Possible side effects at higher doses include muscle or joint pain, constipation, and gout.
~~~~~~~
FINAL NOTE: I will be starting IPE soon. I am hopeful it will improve my symptoms which have been worsening rapidly recently. I will report back! 🐟













Thank you for putting this together! Something else for me to try.
great article, thanks for the summary